Kent E. Dicks, Founder & CEO of Life365, shares his perspective on how telemedicine and virtual care can help address the VA’s growing physician shortage and expand access for veterans.
The proposed Remote Therapeutic Monitoring CPT codes offer new opportunities to bring digital technologies into clinical practice. They can enhance the patient experience, provide additional support to patients between appointments, and arm practitioners with additional data insights.
We provided an overview of the new Remote Therapeutic Monitoring (RTM) CPT codes in a previous blog post that you can read, here. While the Remote Therapeutic Monitoring codes were modeled after the Remote Patient Monitoring (RPM) CPT codes, there are some key differences. One key difference between the RPM and RTM codes are the eligible clinical use cases that qualify for reimbursement.
For a patient to qualify for RPM services, they would need to have one or more acute or chronic conditions. In contrast, the RTM codes limit use case eligibility to the respiratory and musculoskeletal systems only.
As outlined in the proposed fee schedule, the device supply codes for RTM are as follows;
- CPT® code 989X2 - Remote therapeutic monitoring (e.g., respiratory system status, musculoskeletal system status, therapy adherence, therapy response); device(s) supply with scheduled (e.g., daily) recording(s) and/or programmed alert(s) transmission to monitor respiratory system, each 30 days
- CPT® code 989X3 - Remote therapeutic monitoring (e.g., respiratory system status, musculoskeletal system status, therapy adherence, therapy response); device(s) supply with scheduled (e.g., daily) recording(s) and/or programmed alert(s) transmission to monitor musculoskeletal system, each 30 days
989X2 is for transmissions to monitor the respiratory system, and 989X3 is for transmissions to monitor the musculoskeletal system; no other biological systems were included in the code descriptors.
While the clinical use cases of RTM are limited, the types of data that can be captured for RTM is more broad than RPM, and offers more applications for use in patient care. RPM data capture is limited to devices that capture physiologic data, while RTM is for medical devices / applications that capture non-physiologic data, like medication therapy adherence, and therapy response. The use of “e.g.” in the code descriptors indicates there are other examples of data that can be captured that were not specifically mentioned in the list.
We have included some illustrations of how RTM might be utilized in practice.
Remote Therapeutic Monitoring Use Case Examples
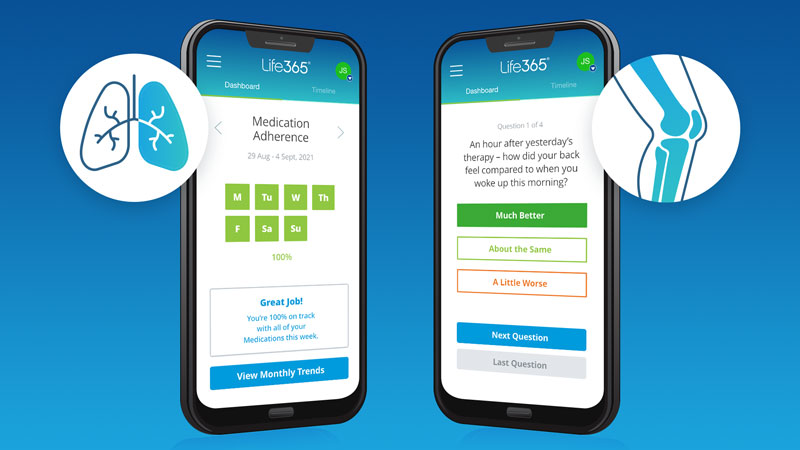
Medication Adherence
A patient with COPD is provided an application that can be downloaded on their personal mobile phone to assist with medication reminders and to help track medication adherence. The application informs the practitioner of medication adherence levels and whether the medication was taken at the appropriate time. If a patient is struggling to adhere to the regimen - the provider can explore ways to modify the regimen, or improve adherence.
Therapy Adherence
A patient with asthma is provided breathing exercises to practice at home for 5-10 minutes per day. The patient downloads an application that walks them through the exercise therapy, and sends adherence updates to the care team.
Symptom Assessment
A patient with COPD is sent a daily survey to complete. They are asked to track their daily symptoms, and share how they feel from day to day. This can allow the patient, provider, and care team the opportunity to discuss changes in the patient’s health status together.
Pain Assessment
An individual completing physiotherapy is provided a self report pain assessment to complete daily. The assessment helps track the location, duration, intensity and etiology of their pain. The therapist is provided valuable feedback to help evaluate the efficacy of pain treatment.
Technology for RTM
CMS clarified in the proposed rule that non-physiologic data may include self reported data. This data is not required to be captured by an actual physical device, but can be captured through other means such as from mobile apps, or web-based platforms. These latter tools are more likely to be used to capture metrics like mood and pain levels.
RTM still requires the use of a medical device as defined by the FDA. The RTM rules allow for self reported data, which would / could be captured from non physical medical devices, like software and apps, termed “Software as a Medical Device.” The term Software as a Medical Device is defined by the International Medical Device Regulators Forum (IMDRF) as "software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device." You can read more about Software as a Medical Device (SaMD) on the FDA website, here.
Summary
The proposed rule greatly expands the potential for use of digital technologies in patient care, but there are still many questions left unanswered in the proposed ruling. Feedback is being provided to CMS from stakeholders interested in RTM, and we expect more clarity by the end of the year as the codes become more defined, and the final rule is published.
Latest Resources
Stay informed with our latest news, resources, and insights!
-1.png)

-1.png)
-1.png)